1,4-Benzenedimethanethiol (1,4-BDMT) as a scavenger for greener peptide resin cleavages†
Abstract
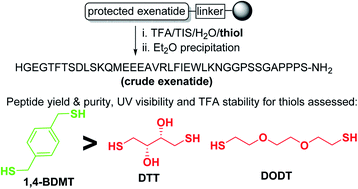
Aiming at elevating the environmental profile of the solid-phase peptide synthesis (SPPS) methodology by improving the quality of crude peptides that SPPS provides we assessed a series of benzylthiols (BTs) as scavengers for global deprotection/TFA cleavage of exenatide peptide resin accessed by Fmoc SPPS. In these studies we identified 1,4-BDMT as a scavenger that affords the peptide in higher quality than the standard aliphatic thiol reagents, not least in terms of the content of critical peptide impurities in the crude material. Further, 1,4-BDMT exhibited favorable UV detectability as well as stability and solubility in TFA. Finally, based on the MS assessment of the crude exenatide products herein we propose that thiol scavengers in the cleavage of Trp containing peptide resins do not minimize the content of Trp oxidants by means of inhibiting Trp oxidation but rather by forming a peptide–thiol adduct via a mechanism involving an attack of a thiol on an oxindolylalanine (Oia) impurity present in the crude material.


 Please wait while we load your content...
Please wait while we load your content...