Novel base-initiated cascade reactions of hemiindigos to produce dipolar γ-carbolines and indole-fused pentacycles†
Abstract
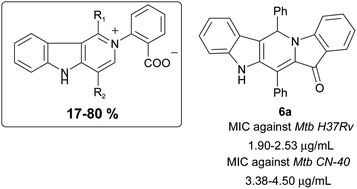
Novel continuous-flow cascade reactions are developed for producing 1,4-diaryl-disubstituted dipolar γ-carbolines 2 that contain a carboxylate group and their two pentacyclic precursors 6, 7 from hemiindigos 1. The nucleophilic and pro-electrophilic chemistry described is new to the hemiindigos 1, and it led to the discovery of antimycobacterial scaffold characteristic of rimino-type pentacycles 6, 7 and potent drug clofazimine. The new scaffold like clofazimine appears to be useful in developing lead agents active against drug-resistant/dormant TB.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives


 Please wait while we load your content...
Please wait while we load your content...