Mono and co-immobilization of imidazolium ionic liquids on silica: effects of the substituted groups on the adsorption behavior of 2,4-dinitrophenol†
Abstract
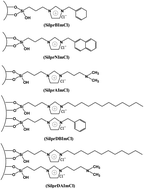
Ionic liquid modified silicas with high adsorption capacity for phenols prompt us to deeply explore the contribution of interactions between the adsorbent and adsorbate, with a particular focus on hydrophobicity, π–π, electrostatic and acid–base interactions. Herein, by introducing a series of typical substituent groups including N,N-dimethylaminopropyl (A), benzyl (B), dodecyl (D) and naphthylmethyl (N) in an imidazole ring (Im), three mono-immobilized and two co-immobilized imidazolium ionic liquid modified silicas, namely SilprAImCl, SilprBImCl, SilprNImCl, SilprDBImCl and SilprDAImCl, werre synthesized for removal and recovery of 2,4-dinitrophenol (2,4-DNP) from aqueous solutions. Adsorption kinetics, isotherms, thermodynamic analysis and desorption experiments have been carried out. The experimental results reveal that the substituent groups such as N,N-dimethylaminopropyl, benzyl and naphthylmethyl on the imidazole ring can significantly enhance the adsorption of 2,4-DNP via the acid–base interaction or π–π interaction and the adsorption capacity of 2,4-DNP follows the order: SilprNImCl > SilprAImCl > SilprBImCl. Furthermore, SilprDBImCl exhibits the largest adsorption capacity and SilprDAImCl has the lowest among the five adsorbents. These interesting finds indicate that the combination of hydrophobicity and π–π interactions lead to enhanced adsorption performance towards 2,4-DNP, while the combination of the hydrophobicity and acid–base interactions can restrain greatly adsorption of 2,4-DNP from aqueous medium. Adsorption mechanisms of 2,4-DNP on the five adsorbents have been clarified. These results will provide a deeper insight for efficient removal of phenols from water environments.


 Please wait while we load your content...
Please wait while we load your content...