Synthesis and reactivity of 2-thionoester pyrroles: a route to 2-formyl pyrroles†
Abstract
2-Functionalised pyrroles exhibit considerable synthetic utility. Herein, the synthesis and reactivity of 2-thionoester (–C(S)OR) pyrroles is reported. 2-Thionoester pyrroles were synthesised using a Knorr-type approach from aliphatic starting materials. 2-Thionoester pyrroles were reduced to the corresponding 2-formyl pyrroles, or the deuterated formyl variant, in one step using RANEY® nickel, thereby removing the need for the much-utilised hydrolysis/decarboxylation/formylation steps that are typically required to convert Knorr-type 2-carboxylate pyrroles into 2-formyl pyrroles. 2-Thionoester pyrroles proved tolerant of typical functional group interconversions for which the parent 2-carboxylate pyrroles have become known.
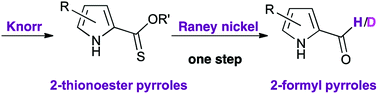


 Please wait while we load your content...
Please wait while we load your content...