Protein–lipid complexes: molecular structure, current scenarios and mechanisms of cytotoxicity
Abstract
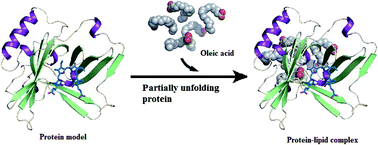
Some natural proteins can be complexed with oleic acid (OA) to form an active protein–lipid formulation that can induce tumor-selective apoptosis. The first explored protein was human milk α-lactalbumin (α-LA), called HAMLET when composed with OA in antitumor form. Several groups have prepared active protein–lipid complexes using a variety of approaches, all of which depend on target protein destabilization or direct OA–protein incubation to alter pH to acid or alkaline condition. In addition to performing vital roles in inflammatory processes and immune responses, fatty acids can disturb different metabolic pathways and cellular signals. Therefore, the tumoricidal action of these complexes is related to OA rather than the protein that keeps OA in solution and acts as a vehicle for transferring OA molecules to tumor cells. However, other studies have suggested that the antitumor efficacy of these complexes was exerted by both protein and OA together. The potential is not limited to the anti-tumor activity of protein–lipid complexes but extends to other functions such as bactericidal activity. The protein shell enhances the solubility and stability of the bound fatty acid. These protein–lipid complexes are promising candidates for fighting various cancer types and managing bacterial and viral infections.


 Please wait while we load your content...
Please wait while we load your content...