Conversion of methane to C2 and C3 hydrocarbons over TiO2/ZSM-5 core–shell particles in an electric field†
Abstract
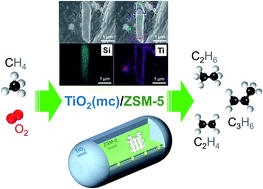
Catalytic conversion of methane (CH4) to light olefins is motivated by increasing recoverable reserves of methane resources, abundantly available in natural gas, shale gas, and gas hydrates. The development of effective processes for conversion of CH4 to light olefins is still a great challenge. The interface of ZSM-5 zeolite and TiO2 nanoparticles is successfully constructed in their core–shell particles via mechanochemical treatment with high shear stress. The oxidative coupling of methane at a low temperature under application of an electric field may be induced by the O2 activation via electrons running through the surface of TiO2 located at the interface of TiO2 and zeolite particles. Moreover, C3H6 was also produced by the ethylene to propylene (ETP) reaction catalyzed by Brønsted acid sites in the ZSM-5 zeolite within core–shell particles.


 Please wait while we load your content...
Please wait while we load your content...