Axially chiral 1,4-dihydropyridine derivatives: aggregation-induced emission in exciplexes and application as viscosity probes†
Abstract
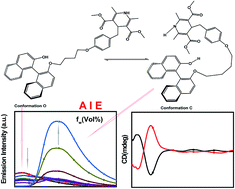
By combining the fluorophores of axially chiral 1,1′-binaphthol (BINOL) and 1,4-dihydropyridine derivatives, axially chiral 1,4-dihydropyridine derivatives ((R)-/(S)-2) with aggregation-induced emission (AIE) in exciplexes were designed and synthesized. (R)-/(S)-2 emitted low fluorescence in THF solutions of their locally excited states; however, they emitted red-shifted fluorescence in the aggregate state upon exciplex formation. Moreover, (R)-/(S)-2 showed linear and multi-exponential relationships between their local excited and exciplex fluorescence intensities and the viscosity of the medium, which allowed us to determine the viscosities of different mixed solvents. In addition, as an axially chiral viscosity probe, (R)-/(S)-2 show excellent CD signals and have potential applications in the fields of chiral recognition and fluorescence imaging, which will broaden the new family of AIE fluorophores. To the best of our knowledge, there are few reports of axially chiral intramolecular exciplex-mediated AIE molecules.


 Please wait while we load your content...
Please wait while we load your content...