Thermal stability, electrochemical and structural characterization of hydrothermally synthesised cobalt ferrite (CoFe2O4)
Abstract
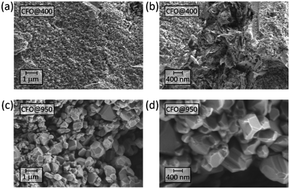
Monophasic nano-crystalline CoFe2O4 (CFO) nanoparticles of high purity have been synthesised through a low temperature hydrothermal route, which does not involve hazardous chemicals, or conditions. The easy, green procedure involves a hydrothermal treatment at 135 °C of an aqueous suspension of the oxalate salts of the precursors. No further purification or annealing procedure was necessary to obtain the crystalline nano-structured oxide. The nanoparticles were characterized structurally and chemically by powder X-ray diffraction (PXRD), Inductively Coupled Plasma Spectrometry (ICP-MS) and Scanning Electron Microscopy (SEM), thus confirming the successful synthesis of the CoFe2O4 particles with the expected crystal phase and stoichiometry and an almost complete inverse spinel structure. From the nanoparticles pellets were pressed to investigate the electronic conduction properties using electrochemical impedance spectroscopy (EIS). At low temperatures, the conductivity measurements reveal a semiconducting behavior originating from hopping between Co sites and a total conductivity dominated by the grain boundary contribution. At higher temperatures (T > 400 °C) a metallic–insulator transition occurs, which is attributed to additional hopping of electrons between the Fe sites.


 Please wait while we load your content...
Please wait while we load your content...