Iodine-mediated C–N and N–N bond formation: a facile one-pot synthetic approach to 1,2,3-triazoles under metal-free and azide-free conditions†
Abstract
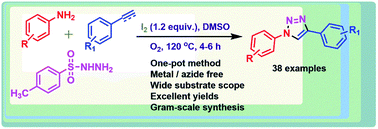
A novel strategy towards the synthesis of 1,4-disubstituted 1,2,3-triazoles via C–N and N–N bond formation has been demonstrated under transition metal-free and azide-free conditions. These 1,2,3-triazoles were obtained in a regioselective manner from commercially available anilines, aryl alkenes/aryl alkynes and N-tosylhydrazines using I2 under O2 atmosphere. Broad substrate scope, milder reaction conditions, good to moderate yields and clean protocol are the notable features of the method. Moreover, this protocol is amenable for the generation of a library of medicinally important key building blocks.


 Please wait while we load your content...
Please wait while we load your content...