Molecular interaction between MeOH and genistein during soy extraction
Abstract
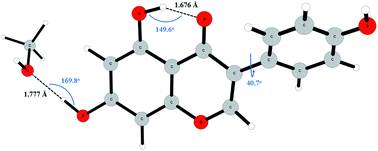
Genistein has received great attention due to its possible anti-oxidant properties. The interaction between genistein and the extraction solvent helps in understanding the extraction efficiency. Hydrogen bonding plays a crucial role in liquid systems. Density functional theory quantum chemical computations in both gas phase and solution were performed to investigate the molecular interaction between genistein and methanol. All the resulting complexes (MeOH : genistein = 1 : 1, 2 : 1, 3 : 1, 6 : 1) were studied using the B3LYP-D3 computational level and the cc-pVTZ basis set. Binding energies demonstrate that more MeOH molecules surrounding genistein could stabilize the system more. Geometry optimizations show that there are strong O–H⋯O interactions between MeOH and genistein. The electron density and the corresponding Laplacian of charge density at bond critical points were also calculated using AIM theory, and the results are in line with the structural and energetic analysis of the studied system. Moreover, energy decomposition analysis shows that the exchange energy term has the largest contribution to the attraction interaction energy as compared with other energy terms. Meanwhile, this study shows that the MeOH–genistein system is more stable under basic conditions. This study could help increase the efficiency of extraction.


 Please wait while we load your content...
Please wait while we load your content...