Synthesis and anion recognition studies of new oligomethylene bis(nitrophenylureylbenzamide) receptors†
Abstract
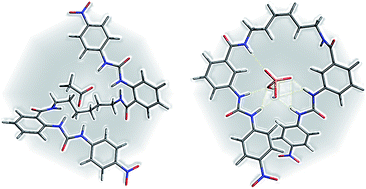
A new series of oligomethylene bis(nitrophenylureylbenzamide) receptors were synthesized varying the relative position of the urea and amide groups (ortho 4 and meta 8) and the length of the oligomethylene chain (C2 to C8). An anion recognition study was performed with TBAX salts (X = AcO−, BzO−, F−, H2PO4−, and HP2O73−) by UV-vis and 1H NMR. The flexibility of these receptors allows a cooperative effect of both ureylbenzamide units in the receptors. Noteworthy, the ortho position favored the 1 : 1 stoichiometry in the complexes with the carboxylates. The formation of 2 : 1 receptor–anion complexes with both types of receptors 4 and 8 and with hydrogen pyrophosphate and high log K values obtained were very significant in this work. The NMR studies evidenced the formation of supramolecular complexes, even in a competitive solvent, such as DMSO.


 Please wait while we load your content...
Please wait while we load your content...