Synthesis, human topoisomerase IIα inhibitory properties and molecular modeling studies of anti-proliferative curcumin mimics†
Abstract
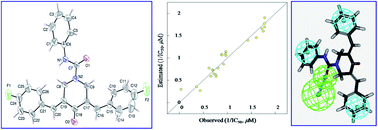
3,5-Bis(arylidene)-N-substituted-4-oxo-piperidine-1-carboxamides 24–51 were synthesized as curcumin mimics in a facile pathway through reaction of 3,5-bis(arylidene)-4-piperidones with the appropriate isocyanate in the presence of triethylamine. The 3E,5E′-stereochemical configuration was conclusively supported by single crystal X-ray studies of compounds 25 and 34. Most of the synthesized piperidinecarboxamides showed high anti-proliferative properties with potency higher than that of 5-fluorouracil (clinically approved drug against colon, breast and skin cancers) through in vitro MTT bio-assay. Some of them revealed anti-proliferative properties at sub-micromolar values (IC50 = 0.56–0.70 μM for compounds 29, 30 and 34–38 against HCT116; and IC50 = 0.64 μM for compound 30 against A431 cell lines) with promising inhibitory properties against human DNA topoisomerase IIα. The safe profile of the anti-proliferative active agents against the RPE1 normal cell line may prove their selectivity towards carcinoma cells. Robust molecular models (2D-QSAR, 3D-pharmacophore) supported the SAR and validated the observed bio-properties.


 Please wait while we load your content...
Please wait while we load your content...