The promoted catalytic hydrogenation performance of bimetallic Ni–Co–B noncrystalline alloy nanotubes†
Abstract
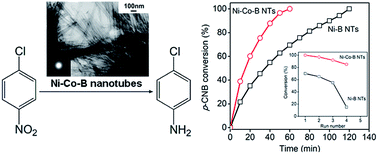
A noncrystalline Ni–B alloy in the shape of nanotubes has demonstrated its superior catalytic performance for some hydrogenation reactions. Remarkable synergistic effects have been observed in many reactions when bimetallic catalysts were used; however, bimetallic noncrystalline alloy nanotubes are far less investigated. Here, we report a simple acetone-assisted lamellar liquid crystal approach for synthesizing a series of bimetallic Ni–Co–B nanotubes and investigate their catalytic performances. The dilution effect of acetone on liquid crystals was characterized by small-angle X-ray diffraction (SAXRD) and scanning electron microscopy (SEM). The Ni/Co molar ratio of the catalyst was varied to study the composition, porous structure, electronic interaction, and catalytic efficiency. In the liquid-phase hydrogenation of p-chloronitrobenzene, the as-prepared noncrystalline alloy Ni–Co–B nanotubes exhibited higher catalytic activity and increased stability as compared to Ni–B and Co–B alloy nanotubes due to electronic interactions between the nickel and cobalt. The excellent hydrogenation performance of the Ni–Co–B nanotubes was attributed to their high specific surface area and the characteristic confinement effects, compared with Ni–Co–B nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...