Mechanistic study on substitution reaction of a citrato(p-cymene)Ru(ii) complex with sulfur-containing amino acids†
Abstract
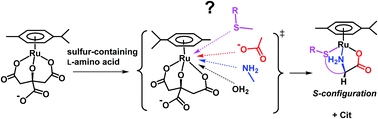
The reactions of a dichloro(p-cymene)ruthenium(II) dimer, [RuCl2(p-cymene)]2, with citric acid and sulfur-containing amino acids gave only [Ru(L)(p-cymene)]-type complexes (L = citrate (Cit), L-penicillaminate (L-Pen), S-methyl-L-cysteinate (S-Me-L-Cys) and L-methioninate (L-Met)) in aqueous solutions at various pHs and molar ratios of the reactants, where Cit and the amino acids act as a tridentate ligand. These sulfur-containing amino acid complexes with bound nitrogen, oxygen and sulfur atoms and η6-p-cymene take S absolute configuration around Ru(II) selectively, having the α-proton oriented in the opposite direction from the Ru(II) center. The concentration dependences of the observed pseudo-first-order rate constants were provided for the substitution reactions of the citrato complex, [Ru(Cit)(p-cymene)], with a large excess of the sulfur-containing amino acids at various temperatures at pH 7.3, where solvolysis path was observed for S-Me-L-Cys and L-Met as an intercept but not for L-Pen. The activation parameters for the substitution reactions by the direct attack of the amino acids were changed significantly, indicating that the reaction mechanism varies sensitively with the amino acids from an associative mechanism to an interchange one. The pH dependences of the rate constants of the substitution reactions suggest that the carboxylate group is an attacking group for S-Me-L-Cys and L-Met under neutral conditions and the thiol group of L-Pen acts as an entering group constantly at any pH showing a considerably smaller activation energy compared with S-Me-L-Cys and L-Met. Differences in stabilities of the amino acid complexes were obtained from the equilibrium constants for the substitution reactions between the amino acids. These results indicate that the activation energies for the substitution reactions of the citrato complex with the amino acids are moderately correlated with the stabilities of the formed amino acid complexes.


 Please wait while we load your content...
Please wait while we load your content...