New α-pyrones from an endophytic fungus, Hypoxylon investiens J2†
Abstract
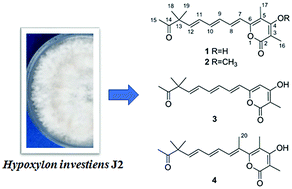
Four new α-pyrones, hypotiens A–D (1–4), were isolated from a fungal endophyte, Hypoxylon investiens J2, harbored in the medicinal plant Blumea balsamifera. Their structures were determined through detailed HRMS and NMR spectroscopic data. Compounds 1–4 are new α-pyrone derivatives containing an unusual dimethyl substitution in the highly unsaturated side chain. Their plausible biosynthetic pathway was discussed. Biological assay indicated that compounds 1–4 showed no antimicrobial, quorum sensing inhibitory, and cytotoxic activities. The specific side chain in α-pyrone derivatives 1–4 might be responsible for the weak pharmacological activities.


 Please wait while we load your content...
Please wait while we load your content...