Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazole Schiff base†
Abstract
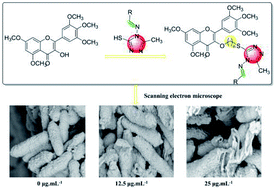
A series of novel myricetin derivatives containing a 1,2,4-triazole Schiff base were designed and synthesized. Their structures were systematically characterized using 1H NMR, 13C NMR, and HRMS. During antibacterial bioassays, 6f, 6i, and 6q demonstrated a good inhibitory effect against Xanthomonas axonopodis pv. citri (Xac), with half-maximal effective concentration (EC50) values of 10.0, 9.4, and 8.8 μg mL−1, respectively, which were better than those of bismerthiazol (54.9 μg mL−1) and thiodiazole copper (61.1 μg mL−1). Note that 6w demonstrated a good inhibitory effect against Ralstonia solanacearum (Rs) with and EC50 value of 15.5 μg mL−1, which was better than those of bismerthiazol (55.2 μg mL−1) and thiodiazole copper (127.9 μg mL−1). Similarly, 6a, 6d, and 6e demonstrated a good inhibitory effect against Xanthomonas oryzae pv. oryzae (Xoo) with EC50 values of 47.1, 61.2, and 61.0 μg mL−1, respectively, which were better than those of bismerthiazol (148.2 μg mL−1) and thiodiazole copper (175.5 μg mL−1). Furthermore, we used scanning electron microscopy (SEM) to study the possible sterilization process of the target compound 6q against Xac. The results indicated the possibility of destroying the bacterial cell membrane structure, resulting in an incomplete bacterial structure, and thus achieving inhibition. Furthermore, antiviral bioassays revealed that most compounds exhibited excellent antiviral activity against tobacco mosaic virus (TMV) at a concentration of 500 μg mL−1. The results of the molecular docking studies for 6g with TMV-CP (PDB code: 1EI7) showed that compound 6g had partially interacted with TMV-CP. Therefore, mechanistic studies of the action of compound 6g could be further studied based on that.


 Please wait while we load your content...
Please wait while we load your content...