Copper-catalyzed direct C–H arylselenation of 4-nitro-pyrazoles and other heterocycles with selenium powder and aryl iodides. Access to unsymmetrical heteroaryl selenides†
Abstract
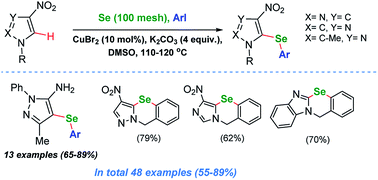
A one-pot, Cu-catalyzed direct C–H arylselenation protocol using elemental Se and aryl iodides was developed for nitro-substituted, N-alkylated pyrazoles, imidazoles and other heterocycles including 4H-chromen-4-one. This general and concise method allows one to obtain a large number of unsymmetrical heteroaryl selenides bearing a variety of substituents. The presence of the nitro group was confirmed to be essential for the C–H activation and can also be used for further functionalisation and manipulation. Several examples of heteroannulated benzoselenazines were also synthesized using the developed synthetic protocol.


 Please wait while we load your content...
Please wait while we load your content...