Tandem grinding reactions involving aldol condensation and Michael addition in sequence for synthesis of 3,4,5-trisubstituted isoxazoles†
Abstract
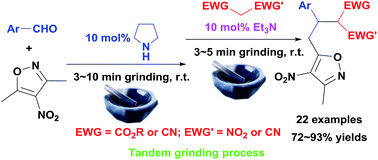
A one-pot, base-catalyzed, tandem grinding process involving carrying out aldol condensation and Michael addition in sequence to produce 3,4,5-trisubstituted isoxazoles from 3,5-dimethyl-4-nitroisoxazole, aromatic aldehydes and activated methylene compounds has been developed. In the presence of 10 mol% of pyrrolidine, aldol condensations of 3,5-dimethyl-4-nitroisoxazole with various aromatic aldehydes were performed with 3–10 minutes of grinding to provide 5-styryl-3-methyl-4-nitroisoxazoles in good to quantitative yields without further purification. Then, Michael additions between 5-styryl-3-methyl-4-nitroisoxazoles and activated methylene compounds (including ethyl 2-nitroacetate and alkyl 2-cyanoacetates) were carried out in the presence of 10 mol% of Et3N in the same mortar with 3–5 minutes of continuous grinding to produce 3,4,5-trisubstituted isoxazoles in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...