Computational investigations of the binding mechanism of novel benzophenone imine inhibitors for the treatment of breast cancer†
Abstract
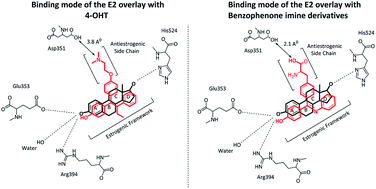
4-Hydroxytamoxifen (4-OHT), the most common hormone used for the treatment of breast cancer, is a selective estrogen receptor modulator (SERM) inhibitor that acts as an antagonist in breast tissue and a partial agonist in the endometrium. However, the detailed molecular mechanism of 4-OHT structure modification has not been well investigated to date. Herein, molecular docking, molecular dynamics simulations and free energy calculations were performed to explore the mechanisms of the molecular interactions between newly designed benzophenone imines (BIs) and the three forms apo, antagonist and agonist of the human estrogen receptor hERα. The proposed inhibitors were designed by replacing the triarylethylene estrogenic scaffold found in 4-OHT with Schiff base triarylimine derivatives. The antiestrogen scaffold i.e. the O-alkyl side chain in 4-OHT was developed by incorporating an alanine amino acid side chain functionality into the triarylimine scaffold. Docking results reveal that the newly designed BIs bind to the hydrophobic open pocket of the apo and antagonist hERα conformations with higher affinity as compared to the natural and synthetic estrogen estradiol (E2) and 4-OHT. The analysis of the molecular dynamics simulation results based on six different systems of the best docked BI (5c) with hERα receptors demonstrates stable interactions, and the complex undergoes fewer conformational fluctuations in the open apo/antagonist hERα receptors as compared to the case of the closed agonist. In addition, the calculated binding free energies indicate that the main factor that contributes to the stabilization of the receptor–inhibitor complexes is hydrophobic interactions. This study suggests that the development of these Schiff base derivatives may be worth exploring for the preparation of new 4-OHT analogues.


 Please wait while we load your content...
Please wait while we load your content...