One-pot sequential synthesis and antifungal activity of 2-(benzylsulfonyl)benzothiazole derivatives†
Abstract
New antifungal agrochemicals, derived from 2-(benzylsulfonyl)benzothiazole were synthesized by an environmentally friendly method, using water as reaction medium. These compounds were prepared by a one-pot, two-step synthesis, starting from 2-mercaptobenzothiazole and benzyl halides. The potential fungicides were tested against a panel of phytopathogenic fungi, with many of them showing a significant improvement compared to the non-oxidized analogues and the commercial antifungal Captan. The new derivatives 2-((2-chlorobenzyl)sulfonyl)benzo[d]thiazole (4f) and 2-((4-methylbenzyl)sulfonyl)benzo[d]thiazole (4k) presented remarkable properties, being able to inhibit the growth of two resistant moulds (Aspergillus fumigatus and Aspergillus ustus). Both 4f and 4k could be classified as broad-spectrum fungicides, emerging as possible candidates for the control of these moulds, which have negative impact in food production.
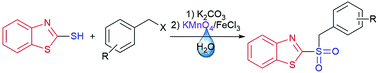
- This article is part of the themed collection: Editors' collection: Food Engineering, Science, Technology, and Nutrition


 Please wait while we load your content...
Please wait while we load your content...