Homogeneous catalytic reduction of polyoxometalate by hydrogen gas with a hydrogenase model complex†
Abstract
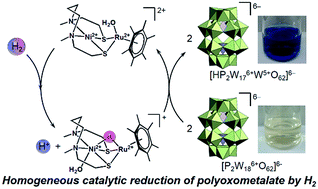
The homogeneous catalytic reduction of a polyoxometalate (POM) by hydrogen gas in aqueous media was investigated for the first time by using a [NiRu] hydrogenase model complex (I) under very mild conditions. By bubbling hydrogen gas into the buffer solution containing I and the Dawson-type POM (IIox), the color of the solution turned from pale yellow to dark blue, suggesting the reduction of IIox. The catalytic and kinetic studies revealed that I acted as an efficient catalyst to yield one-electron-reduced Dawson-type POM (IIred) with a low energy barrier for activating dihydrogen and reducing IIox via a hydride complex of I. The process for the one-electron reduction of IIox was confirmed by UV-vis spectroscopy, controlled potential electrolysis, and X-ray photoelectron spectroscopy. POM IIred could stably store protons and electrons and release them by addition of oxidants, demonstrating that POMs acted as redox active mediators for transporting protons and electrons from hydrogen gas to acceptors. The recycle study showed that IIox and IIred could be reduced and oxidized by hydrogen and oxygen gases, respectively, at least five times with >99% yield of reduced species, showing a durable system for extracting protons and electrons from hydrogen gas.


 Please wait while we load your content...
Please wait while we load your content...