Copper-catalyzed synthesis of phenol and diaryl ether derivatives via hydroxylation of diaryliodoniums†
Abstract
A copper-catalysed hydroxylation of diaryliodoniums to generate phenols and diaryl ethers is reported. This method allows the synthesis of diversely functionalized phenols under mild reaction conditions without the need for a strong inorganic base or an expensive noble-metal catalyst. Significantly, convenient application of diaryliodoniums is demonstrated in the preparation of diaryl ethers in a one-pot operation.
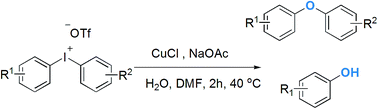


 Please wait while we load your content...
Please wait while we load your content...