Rational design of MgF2 catalysts with long-term stability for the dehydrofluorination of 1,1-difluoroethane (HFC-152a)†
Abstract
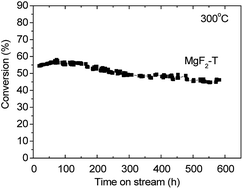
In this study, three different approaches, i.e. the sol–gel method, precipitation method and hard-template method, were applied to synthesize MgF2 catalysts with improved stability towards the dehydrofluorination of hydrofluorocarbons (HFCs); the in situ XRD technique was employed to investigate the relationship between the calcination temperature and the crystallite size of precursors to determine optimal calcination temperature for the preparation of the MgF2 catalysts. Moreover, the physicochemical properties of MgF2 catalysts were examined via BET, XRD, EDS and TPD of NH3 and compared. Undoubtedly, the application of different methods had a significant influence on the surface properties and catalytic performances of MgF2 catalysts. The surface areas of the catalysts prepared by the precipitation method, sol–gel method and template method were 120, 215 and 304 m2 g−1, respectively, upon calcination at 200 °C. However, the surface area of the MgF2 catalysts decreased significantly when the calcination temperatures of 300 and 350 °C were applied. The catalytic performance of these catalysts was evaluated via the dehydrofluorination of 1,1-difluoroethane (HFC-152a). The MgF2 catalyst prepared by the precipitation method showed the lowest catalytic activity among all the MgF2 catalysts. When the calcination temperature was above 300 °C, the MgF2 catalysts prepared via the template method demonstrated the highest catalytic conversion rate with catalytic activity following the order: MgF2-T (template method) > MgF2-S (sol–gel method) > MgF2-P (precipitation method). The conversion rate generally agreed with the total amount of acid on the surface of the catalysts, which was measured by the NH3-TPD technique. The MgF2-T catalysts were further examined for the dehydrofluorination of HFC-152a for 600 hours, and a conversion rate greater than 45% was maintained, demonstrating superior long-term stability of these catalysts.


 Please wait while we load your content...
Please wait while we load your content...