A titanium tetrachloride-based effective methodology for the synthesis of dipeptides†
Abstract
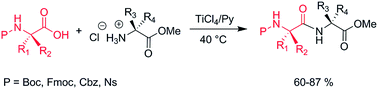
A series of dipeptide systems have been easily achieved through a TiCl4-assisted condensation reaction. The reaction of N-protected amino acids with amino acid methyl esters in pyridine and in the presence of TiCl4 furnished the corresponding dipeptides with high yields and diastereoselectivity. The reaction was successfully applied to amino acids protected on the α-amino function with different protecting groups. The adopted experimental conditions allowed preserving both the protecting groups on the α-amino function and on the side chain functionalities. Furthermore, the preservation of the stereochemical integrity at the amino acid chiral centres has been verified.


 Please wait while we load your content...
Please wait while we load your content...