A novel water-soluble naked-eye probe with a large Stokes shift for selective optical sensing of Hg2+ and its application in water samples and living cells†
Abstract
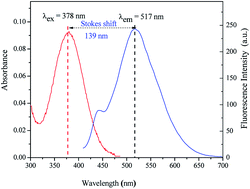
A water-soluble and colorimetric fluorescent probe with a large Stokes shift (139 nm) for rapidly detecting Hg2+, namely Hcy-mP, was synthesized by using an indole derivative and 2,4-dihydroxybenzaldehyde as starting materials. This probe demonstrates good selectivity for Hg2+ over other metal ions including Ag+, Pb2+, Cd2+, Cr3+, Zn2+, Fe3+, Co2+, Ni2+, Cu2+, K+, Na+, Mg2+, and Ca2+ in aqueous solution. With the increase in concentration of Hg2+, the color of the solution changed from pale yellow to pink and the fluorescence intensity decreased slightly. When 5-equivalents of EDTA were added to the solution with Hg2+, the fluorescence intensity of this probe was restored. The probe has been applied to the detection of Hg2+ in real water samples. Moreover, this probe was confirmed to have low cytotoxicity and excellent cell membrane permeability. The effect of Hcy-mP–Hg2+ towards living cells by confocal fluorescence was also investigated.


 Please wait while we load your content...
Please wait while we load your content...