Two zinc(ii) coordination polymers based on flexible co-ligands featuring assembly imparted sensing abilities for Cr2O72− and o-NP†
Abstract
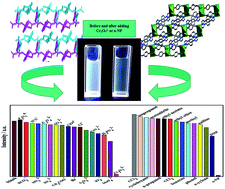
Two new Zn(II) coordination complexes, formulated as [Zn(opda)(pbib)] (1) and [Zn(ppda)(pbib)(H2O)] (2), (H2opda = 1,2-phenylenediacetic acid, H2ppda = 1,4-phenylene-diacetic acid, pbib = 1,4-bis(1-imidazoly)benzene), have been synthesized. The opda ligands extend a 1D chain containing (Zn-pbib) polymer chains into a 2D layer in 1. In 2, the ppda ligands link Zn(II) atoms to form a 2D network, then the rigid bis(imidazole) ligands give rise to the 3D structure. The fluorescence property application and mechanisms of two complexes for detecting Cr2O72− and o-NP have been researched. For two complexes, the high quenching percentage in low concentration aqueous solution are 95.75% (Cr2O72−, 1), 95.28% (Cr2O72−, 2) and 97.56% (o-NP, 1), 96.59% (o-NP, 2). Compared with 2, complex 1 has higher quenching percentage, this could be because 1 is a 3D supramolecular with a large hole. The detection limits have been measured to be 2.992 × 10−7 M (Cr2O72−, 1), and 4.372 × 10−7 M (Cr2O72−, 2), 2.103 × 10−7 M (o-NP, 1), 1.862 × 10−7 M (o-NP, 2), respectively. The emissions of two complexes could be effectively and selectively quenched by o-NP and Cr2O72−, showing their potential as multi-responsive luminescent sensors.


 Please wait while we load your content...
Please wait while we load your content...