Surface protolytic property characterization of hydroxyapatite and titanium dioxide nanoparticles†
Abstract
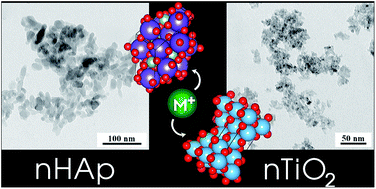
We provide characterization data of hydroxyapatite (nHAp) and titanium dioxide (nTiO2) nanoparticles as potential materials for ion sorption, e.g. in targeted therapy, barrier materials for waste repositories or photovoltaics. The study is focused on the determination of the values of protonation and ion exchange constants and site densities (∑SOH, ∑X; [mol kg−1]) of nTiO2 and nHAp for further Ra kinetics and sorption experiments. These data are very important for further investigation of the materials, which can be used e.g. as drug delivery systems or in engineered barriers of deep geological repositories. The characterization was based on the evaluation of the dependence of titrating agent consumption on pH. Titration results were evaluated on the basis of several model combinations, however the combination of the Chemical Equilibrium Model (CEM) and Ion Exchange Model (IExM) fits best to the experimental titration curves. However, the differences between the two sorbents were relatively large. Due to stability in a broad pH range and available surface sites, nTiO2 seems to have a wide application range. The applicability of nHAp is not so wide because of its dissolution under pH 5. Both sorbents are virtually able to sorb cationic species on deprotonated edge and layer sites with different capacities, which can be important for sorption and decontaminating applications.


 Please wait while we load your content...
Please wait while we load your content...