Palladium catalysed carbonylation of 2-iodoglycals for the synthesis of C-2 carboxylic acids and aldehydes taking formic acid as a carbonyl source†
Abstract
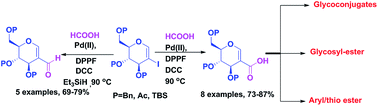
Pd catalyzed carbonylative reaction of 2-iodo-glycals has been developed taking formic acid as a carbonyl source for the synthesis of 2-carboxylic acids of sugars by the hydroxycarbonylation strategy. The methodology was successfully extended to the synthesis of 2-formyl glycals by using a reductive carbonylation approach. Both ester and ether protected glycals undergo the reaction and furnished sugar acids in good yield which is otherwise not possible by literature methods. The C-2 sugar acids were successfully utilized for the construction of 2-amido glycals, 2-dipeptido-glycal by Ugi reaction and C-1 and C-2 branched glycosyl esters.


 Please wait while we load your content...
Please wait while we load your content...