Chemical constituents from the stems of Machilus philippinensis Merr. and the neuroprotective activity of cinnamophilin†
Abstract
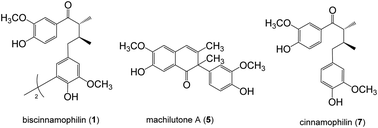
The Machilus genus (Lauraceae) had been extensively utilized in folk medicine due to its broad range of bioactivities. In the present study, a series of chromatographic separations of the methanol extract of stems of M. philippinensis led to the identification of thirty eight compounds totally. Among these, biscinnamophilin (1), machilupins A–C (2–4), machilutone A (5), and machilusoxide A (6) were new compounds reported for the first time. In addition, 5 was characterized with a unprecedented carbon skeleton. Other known compounds, including the major compounds cinnamophilin (7) and meso-dihydroguaiaretic acid (8), are identified by comparison of their physical and spectroscopic data with reported values. One of the reported compounds, cinnamophilin A (10), should be revised as dehydroguaiaretic acid (9) after careful comparison of all the 1H and 13C NMR data. Moreover, the neuroprotective activity of cinnamophilin (7) was examined in a primary cortical neuron culture and the results indicated that 7 was effective against glutamate induced excitotoxicity.


 Please wait while we load your content...
Please wait while we load your content...