Monometallic and bimetallic Cu–Ag MOF/MCM-41 composites: structural characterization and catalytic activity
Abstract
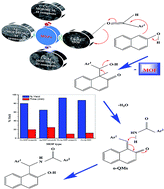
Monometallic and bimetallic MOF/MCM-41 composites (Cu, Ag and Cu–Ag) were synthesized via a solvothermal method. The synthesized composites were characterized by XRD, FTIR, SEM, EDX and BET surface area measurements. The acidity was determined through two techniques; potentiometric titration with n-butyl amine for determining the strength and the total number of acid sites and FTIR spectra of chemisorbed pyridine on the surface of MOFs for determining the type of acid sites (Brønsted and/or Lewis). All the prepared MOFs showed Lewis-acid sites and the higher acidity was observed for the bimetallic Cu–Ag MOF/MCM-41 composite. The catalytic activity was examined on the synthesis of 1-amidoalkyl-2-naphthol via the reaction of benzaldehyde, 2-naphthol and benzamide. The best yield (92.86%) was obtained in the least time (10 min) with a molar ratio 1.2 : 1.2 : 1.7 of benzaldehyde : β-naphthol : benzamide and 0.1 g bimetallic Cu–Ag MOF/MCM-41 composite under solvent-free conditions at 130 °C. Reuse of the catalysts showed that they could be used at least four times without any reduction in the catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...