A new iron-based metal–organic framework with enhancing catalysis activity for benzene hydroxylation†
Abstract
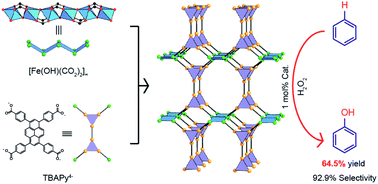
A new Fe-based metal–organic framework (MOF), termed Fe-TBAPy Fe2(OH)2(TBAPy)·4.4H2O, was solvothermally synthesized. Structural analysis revealed that Fe-TBAPy is built from [Fe(OH)(CO2)2]∞ rod-shaped SBUs (SBUs = secondary building units) and 1,3,6,8-tetrakis(p-benzoate)pyrene (TBAPy4−) linker to form the frz topological structure highlighted by 7 Å channels and 3.4 Å narrow pores sandwiching between the pyrene cores of TBAPy4−. Consequently, Fe-TBAPy was used as a recyclable heterogeneous catalyst for benzene hydroxylation. Remarkably, the catalysis reaction resulted in high phenol yield and selectivity of 64.5% and 92.9%, respectively, which are higher than that of the other Fe-based MOFs and comparable with those of the best-performing heterogeneous catalysts for benzene hydroxylation. This finding demonstrated the potential for the design of MOFs with enhancing catalysis activity for benzene hydroxylation.


 Please wait while we load your content...
Please wait while we load your content...