Neodymium β-diketonate showing slow magnetic relaxation and acting as a ratiometric thermometer based on near-infrared emission†
Abstract
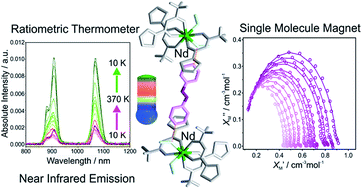
Self-assembly of β-diketonate (Htta = thenoyl(trifluoro)acetone) and 4,4′-azopyridine (Azo-py) with neodymium(III) ions in the presence of methanol resulted in the formation of mononuclear complex [NdIII(TTA)3(MeOH)2]·0.5Azo-py (A) in which two asymmetric units are linked by Azo-py through hydrogen bonding via methanol. A reveals near-infrared emission (NIR) centred at about 895 and 1056 nm, in the 10–370 K temperature range, originating from the two emissive transitions on Nd(III) from 4F3/2 to 4I9/2 and 4I11/2 levels, respectively. Furthermore, the NIR luminescence intensity of A at room temperature augments two times upon thermal elimination of one coordinated methanol molecule. The thermally activated A exhibits single centre ratiometric thermometer behaviour in a wide temperature range from 10 to 300 K. Moreover, fluorescence properties of A were compared to another mononuclear complex [NdIII(TTA)3(4-OHpy)(H2O)] (B). Assembly A also exhibits field-induced slow magnetic relaxation properties with an energy barrier of ΔE/kB = 19.7(7) K and an attempt time of relaxation, τ0 = 3.7(8) × 10−7 s for fresh sample A, and ΔE/kB = 27.3 K and τ0 = 8.5(0) × 10−8 for assembly A after thermal treatment at 370 K.


 Please wait while we load your content...
Please wait while we load your content...