Validated liquid chromatography tandem mass spectrometry for simultaneous quantification of foretinib and lapatinib, and application to metabolic stability investigation
Abstract
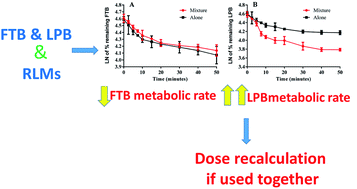
Foretinib (GSK1363089, FTB) is a multikinase inhibitor that inhibits multiple receptor tyrosine kinases, including vascular endothelial growth factor receptor-2 and mesenchymal–epithelial transition factor, with the potential for solid tumor treatment. Lapatinib (LPB) is a significant promising drug molecule that was approved by the USFDA and was utilized to develop a nontoxic and very efficient targeted therapy against breast cancer. There is an ongoing clinical trial for using of FTB and LPB combination for HER-2 positive metastatic breast cancer treatment. In the current study, liquid chromatography tandem mass spectrometry methodology was validated for simultaneous estimation of FTB and LPB with application to drug metabolic stability investigation. Chromatographic separation of FTB, LPB and masitinib (internal standard) was attained using an isocratic mobile phase running on a reversed-phase C18 column. The linear dynamic range was 5–500 ng mL−1 with r2 ≥ 0.9999 in the rat liver microsomes (RLMs) matrix. The FTB and LPB metabolic stabilities in the RLMs matrix were estimated by computing two parameters, intrinsic clearance (CLint: 6.33 and 5.63 mL min−1 kg−1) and a low in vitro half-life (t1/2: 23.9 and 26.9 min), which revealed the FTB and LPB high clearance by the liver from the blood. This probably revealed the low in vivo bioavailability that verified the low oral bioavailability previously reported and also indicated that FTB and LPB will not bioaccumulate after multiple doses. FTB metabolic rate is slightly decreased in combination with LPB, while LPB metabolic rate is greatly increased in combination with FTB. So dose recalculation must be evaluated when FTB and LPB are used in combination.


 Please wait while we load your content...
Please wait while we load your content...