Synthesis and evaluation of antimicrobial activity, cytotoxic and pro-apoptotic effects of novel spiro-4H-pyran derivatives†
Abstract
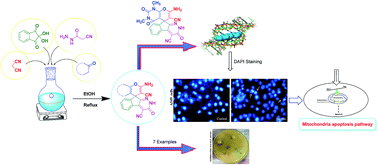
A new library of spiropyrans were synthesized via a one-pot four component reaction of cyanoacetohydrazide, ninhydrin, malononitrile and various cyclic CH-acids in EtOH at reflux conditions. The products were isolated and tested in vitro for antibacterial effects on Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Furthermore cytotoxic activity of the spiropyrans on non-small-cell lung cancer cells (A549 cells), a breast epithelial cancer cell line (MCF-7), human malignant melanoma cells (A375), prostate cancer cells (PC3 cells, LNCaP cells) and normal cells HDF (human dermal fibroblast) was investigated. Interestingly, it was found that compounds 5a, 5b, 5f, 5g and 5i have the best MIC against S. auerus and compound 5a displayed the most potent activity against A549, A375, and LNCaP tumor cells. Moreover, DAPI staining of the A549 cancer cell lines that were treated with 5a were associated with the death of A549 cells. By using RT-PCR method, it was finally confirmed that apoptosis occurs in A549 cells by up-regulated Bax expression and down-regulated Bcl-2 expression from the mitochondrial pathway of apoptosis.


 Please wait while we load your content...
Please wait while we load your content...