Global ab initio exploration of potential energy surfaces for radical generation in the initial stage of benzene oxidation†
Abstract
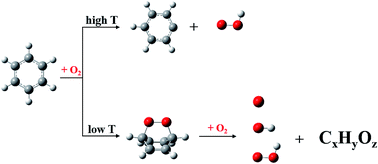
The potential energy surfaces (PESs) of benzene oxidation by molecular oxygen were explored using the anharmonic downward distortion following (ADDF) and artificial force induced reaction (AFIR) methods of the global reaction route mapping (GRRM) strategy. The reaction mechanism of benzene activation by initial molecular oxygen depends on the combustion temperature. At high temperature, the benzene molecule could be oxidized by abstracting hydrogen atoms and form the radical fragments, C6H5 and OOH. However, before reaching its auto-ignition point, the formation of a singlet bridging peroxide molecule C6H6O2 from the triplet reactants via electronic non-adiabatic transition will play a critical role in the increase of the combustion temperature by the generation of initial free radicals. Bridging peroxide C6H6O2 could isomerize to other stable isomers by a consecutive series of oxygen and hydrogen atom transfers. Importantly, these C6H6O2 isomers are vital sources of free radical generation in the initial stage of benzene oxidation. Free radicals, such as OOH, O, and OH, could be generated during the further oxidation of these oxygenated hydrocarbon species C6H6O2 due to the presence of active groups or sp3-C–H bonds.


 Please wait while we load your content...
Please wait while we load your content...