Energy-saving electrolytic γ-MnO2 generation: non-noble metal electrocatalyst gas diffusion electrode as cathode in acid solution
Abstract
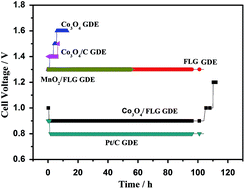
γ-MnO2, which is commercially used as an electrode material in batteries, is produced using large amounts of energy and leads to the production of high pollution as a secondary product. Ideally, this material should be fabricated by energy efficient, non-polluting methods at a reasonable cost. This study reports the green fabrication of γ-MnO2 into a gas diffusion electrode with Pt-free catalysts in acid solution. Cobalt oxide nanoparticles were deposited on few-layer graphene sheets produced via a simple sintering and ultrasonic mixing method, leading to the fabrication of cobalt oxide/few-layer graphene. Co3O4 nanoparticles are irregularly shaped and uniformly distributed on the surface of the few-layer graphene sheets. Characterization was conducted by X-ray diffraction, X-ray photoelectron spectroscopy, and field emission scanning electron microscopy. Electrochemical characterization revealed the performance of cobalt oxide/few-layer graphene gas diffusion electrode in an electrolyte of 120 g L−1 manganese sulfate + 30 g L−1 sulfuric acid at 100 A m−2 at 80 °C. The cobalt oxide/few-layer graphene gas diffusion electrode exhibited a lower cell voltage of 0.9 V and higher electric energy savings of approximately 50% compared with traditional cathodes (copper and carbon).


 Please wait while we load your content...
Please wait while we load your content...