Metabolism and pharmacokinetics of medium chain fatty acids after oral administration of royal jelly to healthy subjects
Abstract
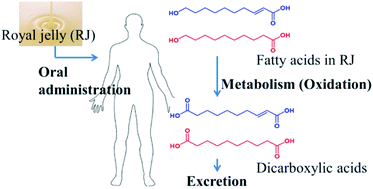
The unique fatty acids in royal jelly (RJ), 10-hydroxy-2-decenoic acid and 10-hydroxydecanoic acid are expected to be associated with many health benefits, but little is known on the pharmacokinetics and metabolism. The aim of this study is to confirm the metabolism and pharmacokinetics of RJ fatty acids in humans. Twelve volunteers received RJ capsules or enzyme treated RJ (ETRJ) capsules (800 mg). The other group received two doses of ETRJ tablets (800 mg and 1600 mg). Plasma samples were collected up to 12 h after the RJ intake and urine samples were collected within 24 h after ETRJ tablet consumption. The samples were analyzed by LC/MS/MS. A multivariate analysis of the RJ dose plasma samples detected 2-decenedioic acid (2-DA), sebacic acid (SA), and 3-hydroxysebacic acid (3-HSA) with significantly different intensities (P < 0.05) before and after RJ intake. The area under the concentration (AUC) of 2-DA, SA, and 3-HSA was 2500.05 ± 569.58, 322.57 ± 137.36, and 242.98 ± 58.36 ng h mL−1, respectively. By enzyme treatment, the AUC of 2-DA, SA, and 3-HSA was significantly increased (P < 0.05). The values of AUC and urinary excretion of these metabolites were dose-dependent. The major RJ fatty acids were metabolized to dicarboxylate, absorbed into the circulation and their absorption increased by enzyme treatment. This study provides useful information that will support studies aimed at clarifying the identity of bioactive RJ constituents and their biological effect, and further the development of RJ.


 Please wait while we load your content...
Please wait while we load your content...