N-doped graphene/CoFe2O4 catalysts for the selective catalytic reduction of NOx by NH3
Abstract
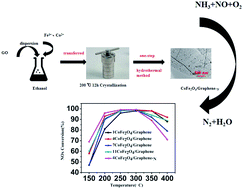
In this paper, CoFe2O4/graphene catalysts and N-doped graphene/CoFe2O4 (CoFe2O4/graphene-N) catalysts were prepared using the hydrothermal crystallization method for the selective catalytic reduction of NOx by NH3. The results of the test showed that CoFe2O4/graphene catalysts exhibited the best denitrification activity when the loading was at 4% and the conversion rate of NOx reached 99% at 250–300 °C. CoFe2O4/graphene-N catalysts presented a better denitrification activity at low temperature than CoFe2O4/graphene catalysts, and the conversion rate of NOx reached more than 95% at 200–300 °C. The intrinsic mechanism of CoFe2O4/graphene-N catalysts in promoting SCR activity was preliminarily explored. The physicochemical properties of the samples were characterized using XRD, TEM, N2 adsorption, XPS, NH3-TPD, and H2-TPR. The results indicated that nitrogen doping can improve the dispersion of CoFe2O4, and it also increased the acidic sites and the redox performance conducive to improving the denitrification activity of the catalysts. In addition, CoFe2O4/graphene-N catalysts demonstrated a better resistance to water and sulfur than CoFe2O4/graphene catalysts.


 Please wait while we load your content...
Please wait while we load your content...