Electrocarboxylation of 1-chloro-(4-isobutylphenyl)ethane with a silver cathode in ionic liquids: an environmentally benign and efficient way to synthesize Ibuprofen†
Abstract
Electrocarboxylation of organic halides is one of the most widely used approaches for valorising CO2. In this manuscript, we report a new greener synthetic route for synthesising 2-(4-isobutylphenyl)propanoic acid, Ibuprofen, one of the most popular non-steroidal anti-inflammatory drugs (NSAIDs). The joint use of electrochemical techniques and ionic liquids (ILs) allows CO2 to be used as a C1-organic building block for synthesising Ibuprofen in high yields, with conversion ratios close to 100%, and under mild conditions. Furthermore, the determination of the reduction peak potential values of 1-chloro-(4-isobutylphenyl)ethane in several electrolytes (DMF, and ionic liquids) and with different cathodes (carbon and silver) makes it possible to evaluate the most “energetically” favourable conditions for performing the electrocarboxylation reaction. Hence, the use of ILs not only makes the electrolytic media greener, but they also act as catalysts enabling the electrochemical reduction of 1-chloro-(4-isobutylphenyl)ethane to be decreased by up to 1.0 V.
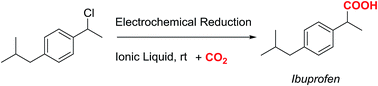


 Please wait while we load your content...
Please wait while we load your content...