Efficient acylation of gastrodin by Aspergillus oryzae whole-cells in non-aqueous media†
Abstract
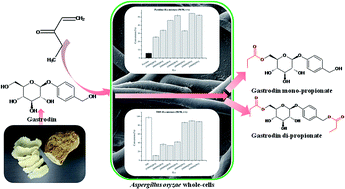
Gastrodin, a bioactive compound extracted from the plant source of Gastrodia elata Blume, has a wide range of therapeutic effects on central nervous system (CNS) diseases, but suffers from poor brain permeability and short half-life in plasma. In this study, fatty acid esters of gastrodin were successfully synthesized by a whole cell-based biocatalytic method. Aspergillus oryzae cells showed different catalytic activities in the organic solvent systems tested. Tetrahydrofuran was confirmed as the most suitable pure organic solvent, with the highest substrate conversion of 98.0%. Addition of ionic liquids (ILs) into pyridine dramatically accelerated the reaction with conversion increased from 5.9% to 84.2%, and also changed the selectivity of the cells, mainly due to the use of IL-containing systems altering cell permeability and contact of the enzymes with solvent molecules possessing different polarities. The ester products were characterized by 13C-NMR and ESI-MS as gastrodin monoester and diester.


 Please wait while we load your content...
Please wait while we load your content...