The nature of the chemical bond in oxyanionic crystals based on QTAIM topological analysis of electron densities †
Abstract
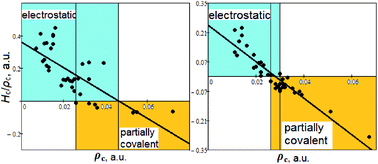
The nature of the chemical bond in 31 oxyanionic crystals was analyzed using ab initio calculations and quantum theory of atoms in molecules (QTAIM). The QTAIM topological analysis of the calculated electron densities in oxyanionic crystals revealed that some of the metal–oxygen, metal⋯metal, metal–ligand and hydrogen bonds are partly covalent in nature. The covalency criteria for metal–oxygen and hydrogen bonds based on electron densities at the bond critical points were obtained. The densities at the bond critical points correlate with the corresponding overlap populations, electronegativities, H-bond lengths and energies. The distances between cationic nuclei and the bond critical points correlate with cationic radii. There are weak anion⋯anion interactions.


 Please wait while we load your content...
Please wait while we load your content...