Synthesis and antiproliferative assay of triazolyl-2,2-dimethyl-3-phenylpropanoates as potential HDAC inhibitors
Abstract
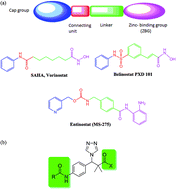
Recently, histone deacetylase (HDAC) inhibition has gained great importance in cancer treatment. We herein, describe the design, synthesis and biological testing of 16 compounds based on the structure modification of methyl 3-(4-(2-chloroacetamido)phenyl)-3-hydroxy-2,2-dimethylpropanoate (5) and methyl 3-(4-chlorophenyl)-3-hydroxy-2,2-dimethylpropanoate (14) as potent HDACIs. Two series were synthesized based on the structure of 3-(4-(2-chloroacetamido)phenyl)-3-hydroxy-2,2-dimethylpropanoate and 3-(4-chlorophenyl)-3-hydroxy-2,2-dimethylpropanoate. The compounds were tested in vitro for their antiproliferative activity against HeLa cells. The results identified compounds 16b, 16c, 18 (IC50; 11.69, 0.69, 3.39 μM respectively) as potential good inhibitors compared to the standard drug doxorubicin (IC50; 2.29 μM). Those compounds also exhibited promising activity against other cancer cell lines namely; HCT-116, MCF-7, PC3, A549 and therefore were selected as hits for further optimization. The docking experiment results performed on the HDAC-2 crystal structure were in close agreement with the biological testing results which suggest that those compounds potentially work through HDAC inhibition.


 Please wait while we load your content...
Please wait while we load your content...