Antibacterial activity and mechanism of action of a thiophenyl substituted pyrimidine derivative†
Abstract
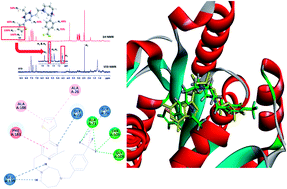
The issue of multidrug resistant bacteria is a worldwide health threat. To develop new antibacterial agents with new mechanisms of action is thus an urgent request to address this antibiotic resistance crisis. In the present study, a new thiophenyl-pyrimidine derivative was prepared and utilized as an effective antibacterial agent against Gram-positive strains. In the tests against MRSA and VREs, the compound showed higher antibacterial potency than that of vancomycin and methicillin. The mode of action is probably attributed to the effective inhibition of FtsZ polymerization, GTPase activity, and bacterial cell division, which cause bactericidal effects. The compound could be a potential candidate for further development as an effective antibiotic to combat drug-resistant bacteria.
- This article is part of the themed collection: Editors' collection: Chemical Biology


 Please wait while we load your content...
Please wait while we load your content...