Effect of moderate magnetic fields on the surface tension of aqueous liquids: a reliable assessment†
Abstract
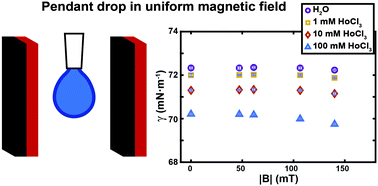
We precisely measure the effect of moderate magnetic field intensity on the surface tension of liquids, by placing pendant drops inside uniform fields where bulk forces due to gradients are eliminated. The surface tension of water is unaffected while that of paramagnetic salt solutions slightly decreases with increasing field strength.


 Please wait while we load your content...
Please wait while we load your content...