Structural evolution of LiNn+ (n = 2, 4, 6, 8, and 10) clusters: mass spectrometry and theoretical calculations†
Abstract
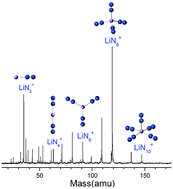
Mixed nitrogen-lithium cluster cations LiNn+ were generated by laser vaporization and analyzed by time-of-flight mass spectrometry. It is found that LiN8+ has the highest ion abundance among the LiNn+ ions in the mass spectrum. Density functional calculations were conducted to search for the stable structures of the Li–N clusters. The theoretical results show that the most stable isomers of LiNn+ clusters are in the form of Li+(N2)n/2, and the order of their calculated binding energies is consistent with that of Li–N2 bond lengths. The most stable structures of LiNn+ evolve from one-dimensional linear type (C∞v, n = 2; D∞h, n = 4), to two-dimensional branch type (D3h, n = 6), then to three-dimensional tetrahedral (Td, n = 8) and square pyramid (C4v, n = 10) types. Further natural bond orbital analyses show that electrons are transferred from the lone pair on Nα of every N2 unit to the empty orbitals of lithium atom in LiN2–8+, while in LiN10+, electrons are transferred from the bonding orbital of the Li–Nα bonds to the antibonding orbital of the other Li–Nα bonds. In both cases, the N2 units become dipoles and strongly interact with Li+. The average second-order perturbation stabilization energy for LiN8+ is the highest among the observed LiNn+ clusters. For neutral LiN2–8 clusters, the most stable isomers were also formed by a Li atom and n/2 number of N2 units, while that of LiN10 is in the form of Li+(N2)3(η1-N4).


 Please wait while we load your content...
Please wait while we load your content...