Investigation of the electrochemical oxidation of 2,3′-bisindolylmethanes in positive-ion electrospray ionization mass spectrometry†
Abstract
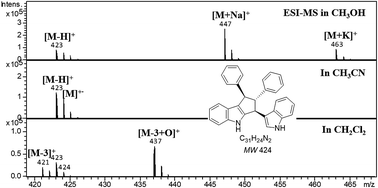
Electrochemical reactions in electrospray may affect the electrospray ionization mass spectrometry (ESI-MS) detection of the analytes. During ESI-MS analysis of 2,3′-bisindolylmethanes in the positive-ion mode, when methanol or acetonitrile was used as the solvent, electrochemical oxidation products as the dehydro and radical cations were observed. Lower sample introduction rates tended to result in a higher oxidation ratio of 2,3′-bisindolylmethanes. The ESI-MS experiments of 2,3′-bisindolylmethanes in different acetonitrile/methanol solvent mixtures indicated that abundant H+, Na+ or K+ would facilitate the adduct formation process, and otherwise electrochemical oxidation dominated the ionization process. Additionally, when dichloromethane was used as the solvent, [M − 3H]+, [M − 2H]+˙ and [M − 3H + O]+ ions are the predominant ions, and the experimental results revealed that the dehydrogenation and oxidative dehydrogenation reactions of 2,3′-bisindolylmethanes in dichloromethane are electrochemical reactions.


 Please wait while we load your content...
Please wait while we load your content...