Synthesis of novel 10,11-methylenedioxy-camptothecin glycoside derivatives and investigation of their anti-tumor effects in vivo†
Abstract
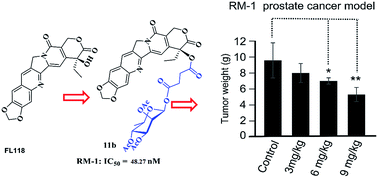
10,11-Methylenedioxy-camptothecin (FL118) is a novel camptothecin analogue that possesses exceptional antitumor efficacy in human tumor xenograft models. The aim of the current study was to develop novel 20-substituted FL118 derivatives coupled with glycosyl-succinic acid esters with improved antitumor efficacy. These FL118 glycoside derivatives were designed, synthesized and their cytotoxicity evaluated in three tumor cell lines (A-549, MDA-MB-231 and RM-1). All of the derivatives showed superior in vitro cytotoxic activity and were more potent than irinotecan in A549 and MDA-MB-231 cells. In mouse prostate cancer cells RM-1, 10,11-methylenedioxy-camptothecin rhamnoside 11b displayed significant activities with IC50 of 48.27 nM. Western blot analysis demonstrated that 11b inhibited survivin expression and induced cancer cells apoptosis. Further cell cycle analyses clearly showed 11b induced G2/M phase cell cycle arrest. Molecule docking studies suggested that the binding mode of 11b was different from that of the crystal complex of ligand topotecan in Top1/DNA. Importantly, 11b showed high in vivo antitumor efficacy in the RM-1 mouse model with transplantation of prostate cancer (TGI = 44.9%) at dose of 9 mg kg−1 without apparent toxicity.


 Please wait while we load your content...
Please wait while we load your content...