Sulfur, mercury, and boron adducts of sydnone imine derived anionic N-heterocyclic carbenes†
Abstract
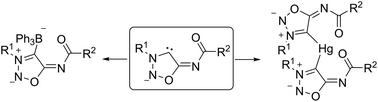
The sydnone imines (5-benzoylimino)-3-(2-methoxyphenyl)-sydnone imine and molsidomine were deprotonated at C4 to give sydnone imine anions which can be represented as anionic N-heterocyclic carbenes, respectively. Trapping reactions with sulfur gave unstable sydnone imine sulfides which were stabilized by the formation of methyl thioethers, methyl sulfoxides, gold complexes [(PPh3)Au–S-sydnone imine] and a bis(ligand) mercury(II) complex. The latter possesses a tetrahedral coordination of the mercury central atom to the sulfur atoms with the N6 nitrogen atoms coordinating as neutral ligands. Water converted the molsidomine anion into ethyl(2-morpholino-2-thioxoacetyl)carbamate. Mercury(II)chloride and triphenylborane were employed to trap the sydnone imine carbenes as mercury complexes as well as BPh3 adducts.


 Please wait while we load your content...
Please wait while we load your content...