Design, synthesis and properties investigation of Nα-acylation lysine based derivatives†
Abstract
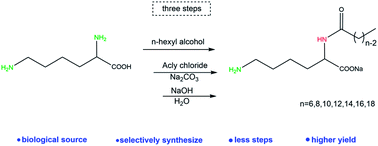
Amino acid-based compounds have attracted attention as environmentally friendly bio-based materials. Our group has recently developed a novel family of Nα-acylation lysine based derivatives. We introduced long chain acyl groups at the Nα position selectively by a new synthetic route that avoided the process of amino protection and deprotection. Sodium Nα-octanamide lysine (C8), sodium Nα-capramide lysine (C10) and sodium Nα-lauramide lysine (C12) can self-assemble into vesicles spontaneously. As a result, not only do they have potential in drug delivery system but also they may be used as bio-based surfactants applied in cosmetics and other industries.


 Please wait while we load your content...
Please wait while we load your content...