Retracted Article: Synthesis of deuterated isopentyl pyrophosphates for chemo-enzymatic labelling methods: GC-EI-MS based 1,2-hydride shift in epicedrol biosynthesis†
Abstract
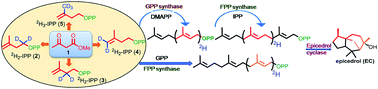
A sesquiterpene epicedrol cyclase mechanism was elucidated based on the gas chromatography coupled to electron impact mass spectrometry fragmentation data of deuterated (2H) epicedrol analogues. The chemo-enzymatic method was applied for the specific synthesis of 8-position labelled farnesyl pyrophosphate and epicedrol. EI-MS fragmentation ions compared with non-labelled and isotopic mass shift fragments suggest that the 2H of C6 migrates to the C7 position during the cyclization mechanism.


 Please wait while we load your content...
Please wait while we load your content...